About Varithena
VARITHENA
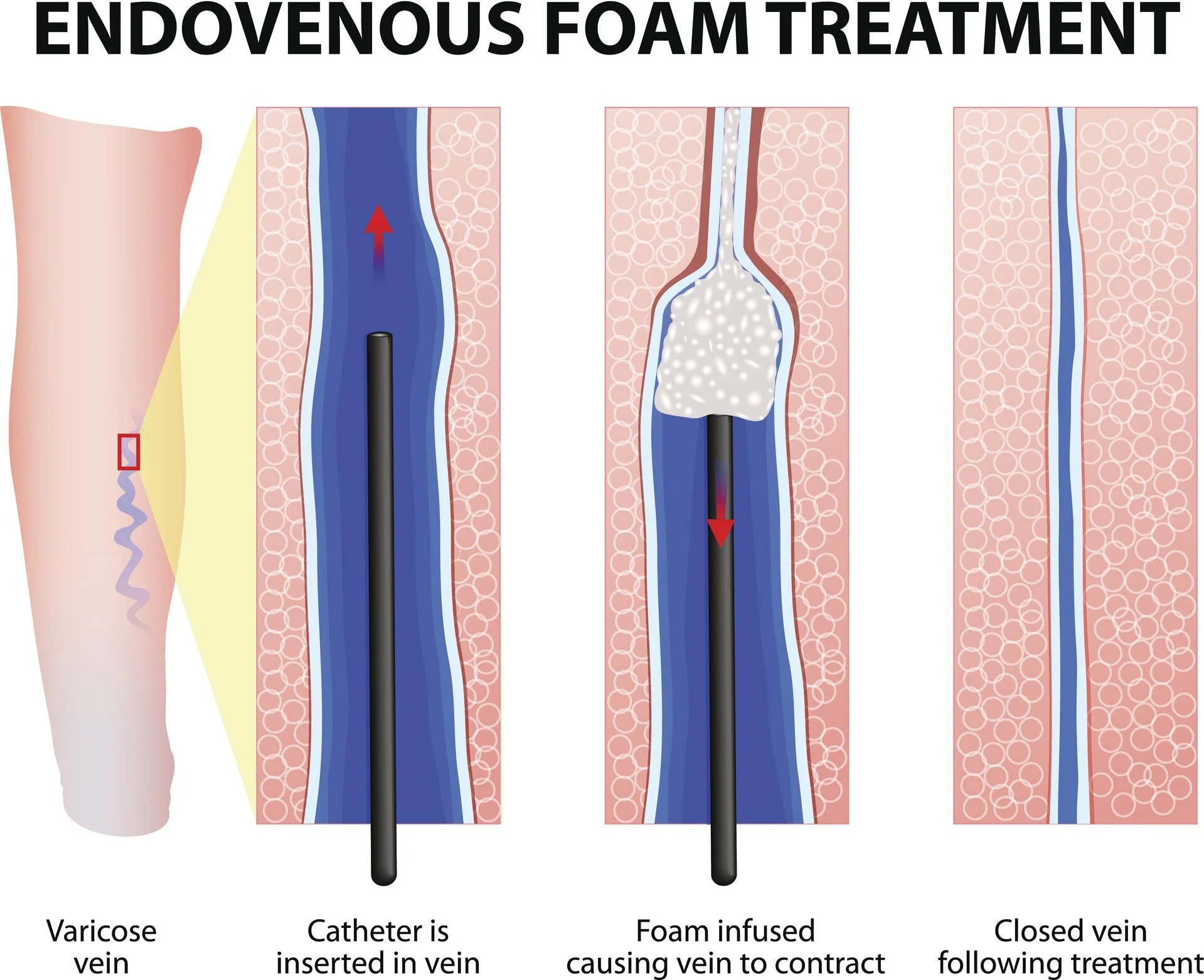
Varithena (polidocanol injectable foam) is indicated for the treatment of incompetent great saphenous veins, accessory saphenous veins, and visible varicosities of the great saphenous vein (GSV) system above and below the knee. Varithena improves the symptoms of superficial venous incompetence and the appearance of visible varicosities.
Varithena is intended for intravenous injection using ultrasound guidance, administered via a single cannula into the lumen of the target incompetent trunk veins or by direct injection into varicosities. Use up to 5 mL per injection and no more than 15 mL per session.
Providers administering Varithena must be experienced with venous procedures and be trained in the administration of Varithena.
Local anesthetic may be administered prior to cannula insertion but neither tumescent anesthesia nor patient sedation is required. Cannulate the vein to be treated using ultrasound guidance to confirm venous access.
A compression stocking will be applied and patient will have to maintain compression for 2 weeks after treatment.
Repeat treatment may be necessary if the size and extent of the veins to be treated require more than 15 mL of Varithena. Separate treatment sessions by a minimum of 5 days